UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported): September 13, 2021
(Exact name of Registrant as specified in its charter)
| (State or Other Jurisdiction of Incorporation or Organization) | (Commission File Number) | (I.R.S. Employer Identification No.) | ||||||
(908 ) 941-1900
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Not Applicable
(Former name or former address, if changed since last report)
________________________________________________________________________________________________________________________
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) | |||||
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) | |||||
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) | |||||
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) | |||||
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. | ||||
Aquestive Therapeutics, Inc. (the “Company”) is furnishing this Current Report on Form 8-K in connection with the disclosure of information, in the form of an investor presentation, to be given at meetings with institutional investors, analysts and others. This information may be amended or updated at any time and from time to time through another Current Report on Form 8-K, a later company filing or other means. A copy of the Company’s investor presentation is attached hereto as Exhibit 99.1 to this Current Report on Form 8-K and incorporated into this Item 7.01 by reference and replaces in its entirety all prior investor presentations filed by the Company. The investor presentation is available on the Company’s website located at www.aquestive.com, although the Company reserves the right to discontinue that availability at any time.
The information in this Item 7.01 (including Exhibit 99.1) shall not be deemed to be “filed” for purposes of, or otherwise subject to the liabilities of, Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), nor shall it be deemed to be incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in any such filing.
| Item 9.01 | Financial Statements and Exhibits | ||||
(d)Exhibits.
| Exhibit Number | Description | |||||||
Investor presentation dated September 2021. | ||||||||
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
Dated: September 13, 2021 | Aquestive Therapeutics, Inc. | |||||||
| By: | /s/ A. Ernest Toth, Jr | |||||||
| Name: A. Ernest Toth, Jr. | ||||||||
| Title: Chief Financial Officer | ||||||||

Advancing medicines. Solving problems. Improving lives. Advancing medicines. Solving problems. Improving lives. September 2021 Aquestive Therapeutics Corporate Presentation

Advancing medicines. Solving problems. Improving lives. Forward-Looking Statement This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “believe,” “anticipate,” “plan,” “expect,” “estimate,” “intend,” “may,” “will,” or the negative of those terms, and similar expressions, are intended to identify forward-looking statements. These forward-looking statements include, but are not limited to, statements regarding the advancement of Libervant™, AQST-108, AQST-109 and other product candidates through the regulatory and development pipeline; and business strategies, market opportunities, and other statements that are not historical facts. These forward-looking statements are subject to the uncertain impact of the COVID-19 global pandemic on our business including with respect to our clinical trials including site initiation, patient enrollment and timing and adequacy of clinical trials; on regulatory submissions and regulatory reviews and approvals of our product candidates; pharmaceutical ingredient and other raw materials supply chain, manufacture, and distribution; sale of and demand for our products; our liquidity and availability of capital resources; customer demand for our products and services; customers’ ability to pay for goods and services; and ongoing availability of an appropriate labor force and skilled professionals. Given these uncertainties, the Company is unable to provide assurance that operations can be maintained as planned prior to the COVID-19 pandemic. These forward-looking statements are based on our current expectations and beliefs and are subject to a number of risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. Such risks and uncertainties include, but are not limited to, risks associated with the Company's development work, including any delays or changes to the timing, cost and success of our product development activities and clinical trials and plans for AQST-108, AQST-109 and our other drug candidates; risk of delays in FDA approval of our drug candidate Libervant and AQST-108, AQST-109 and our other drug candidates or failure to receive approval; ability to address the concerns identified in the FDA’s Complete Response Letter dated September 25, 2020 regarding the New Drug Application for Libervant; risk of our ability to demonstrate to the FDA “clinical superiority” within the meaning of the FDA regulations of Libervant relative to FDA-approved diazepam rectal gel and nasal spray products including by establishing a major contribution to patient care within the meaning of FDA regulations relative to the approved products as well as risks related to other potential pathways or positions which are or may in the future be advanced to the FDA to overcome the seven year orphan drug exclusivity granted by the FDA for the approved nasal spray product of a competitor in the U.S. and there can be no assurance that we will be successful; risk that a competitor obtains FDA orphan drug exclusivity for a product with the same active moiety as any of our other drug products for which we are seeking FDA approval and that such earlier approved competitor orphan drug blocks such other product candidates in the U.S. for seven years for the same indication; risk inherent in commercializing a new product (including technology risks, financial risks, market risks and implementation risks and regulatory limitations); risk of development of our sales and marketing capabilities; risk of legal costs associated with and the outcome of our patent litigation challenging third party at risk generic sale of our proprietary products; risk of sufficient capital and cash resources, including access to available debt and equity financing and revenues from operations, to satisfy all of our short-term and longer term cash requirements and other cash needs, at the times and in the amounts needed; risk of failure to satisfy all financial and other debt covenants and of any default; our and our competitors’ orphan drug approval and resulting drug exclusivity for our products or products of our competitors; short-term and long-term liquidity and cash requirements, cash funding and cash burn; risk related to government claims against Indivior for which we license, manufacture and sell Suboxone® and which accounts for the substantial part of our current operating revenues; risks related to the outsourcing of certain marketing and other operational and staff functions to third parties; risk of the rate and degree of market acceptance of our product and product candidates; the success of any competing products, including generics; risk of the size and growth of our product markets; risks of compliance with all FDA and other governmental and customer requirements for our manufacturing facilities; risks associated with intellectual property rights and infringement claims relating to the Company's products; risk of unexpected patent developments; the impact of existing and future legislation and regulatory provisions on product exclusivity; legislation or regulatory actions affecting pharmaceutical product pricing, reimbursement or access; claims and risks that may arise regarding the safety or efficacy of the Company's products and product candidates; risk of loss of significant customers; risks related to legal proceedings, including patent infringement, investigative and antitrust litigation matters; changes in government laws and regulations; risk of product recalls and withdrawals; uncertainties related to general economic, political, business, industry, regulatory and market conditions and other unusual items; and other uncertainties affecting the Company described in the “Risk Factors” section and in other sections included in our Annual Report on Form 10 K, in our Quarterly Reports on Form 10-Q, and in our Current Reports on Form 8-K filed with the Securities Exchange Commission (SEC). Given those uncertainties, you should not place undue reliance on these forward-looking statements, which speak only as of the date made. All subsequent forward-looking statements attributable to us or any person acting on our behalf are expressly qualified in their entirety by this cautionary statement. The Company assumes no obligation to update forward- looking statements or outlook or guidance after the date of this press release whether as a result of new information, future events or otherwise, except as may be required by applicable law. PharmFilm® and the Aquestive logo are registered trademarks of Aquestive Therapeutics, Inc. All other registered trademarks referenced herein are the property of their respective owners. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy these securities, nor shall there be any sale of these securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. 2

Advancing medicines. Solving problems. Improving lives. Our Path Proven track record of success • Technology-based pharmaceutical company • 5 FDA-approved products • 10+ years of product sales • 200+ patents worldwide 3 Near-term pipeline catalysts • Libervant™ Buccal Film (Diazepam) ‒ NDA filed on June 23, 2021 ‒ PDUFA date December 23, 2021 • Epinephrine prodrug platform with 2 clinical stage sublingual film candidates (SF) AQST-108 & AQST-109 Multiple cash-generating opportunities • 10+ quarters of continuous growth in commercial sales • Cash flow positive manufacturing business • Business performance and capital options support commercial operations, Libervant launch and pipeline development Libervant™ Buccal Film (Diazepam) is an investigational drug being evaluated for use in children and adults with refractory seizures, who remain on stable regimens of antiepileptic drugs, to control bouts of increased seizure activity. The product profile, data from our trials, and related statements have not been approved by the FDA. Aquestive has received conditional acceptance of the use of this trade name, which is subject to final FDA review and acceptance. © 2021 Property of Aquestive Therapeutics, Inc.

Advancing medicines. Solving problems. Improving lives. PharmFilm® Technology – Where You Need It, When You Need It™ 4

Advancing medicines. Solving problems. Improving lives. Our Team Keith J. Kendall Chief Executive Officer and Director Daniel Barber Chief Operating Officer Peter Boyd SVP, Business Process & Information Technology Lori J. Braender General Counsel and Chief Compliance Officer Ken Marshall Chief Commercial Officer Mark Schobel Chief Innovation & Technology Officer Gary H. Slatko, MD Chief Medical Officer, Neurology Ernie Toth Chief Financial Officer Theresa Wood SVP, Human Resources Mark Lepore, MD Chief Medical Officer, Allergy 5

Advancing medicines. Solving problems. Improving lives. Our Products AQST-108-SF (Epinephrine) (Alternate Indications) FDA Approved 2018 SYMPAZAN® Oral Film (Clobazam) (Lennox-Gastaut syndrome) AQST-305-SF (Octreotide) (Acromegaly/Carcinoid Syndrome) AQST-109-SF (Epinephrine) (Anaphylaxis) Proprietary growth drivers Pre-Clinical Clinical Filed Marketed FDA Approved 2010 Suboxone® Sublingual Film (Buprenorphine/Naloxone) (Opioid Dependence) Licensed commercial products Pre-Clinical Clinical Filed Marketed Exservan™ Oral Film (Riluzole) (Amyotrophic Lateral Sclerosis/ALS) FDA Approved 2019 Kynmobi® Sublingual Film (Apomorphine HCI) (OFF Episodes of Parkinson’s) FDA Approved 2020 Zuplenz® Oral Film (Ondansetron) (Antiemetic) FDA Approved 2010 6 LIBERVANT™ Buccal Film (Diazepam) (Refractory Seizures) AzstarysTM (serdexmethylphenidate and dexmethylphenidate) (Attention-Deficit Hyperactivity Disorder/ADHD) FDA Approved 2021 Filed PDUFA 12/23/21 EMA and TGA Approved

Advancing medicines. Solving problems. Improving lives. • Commercialized for Lennox-Gastaut syndrome (LGS), a rare, severe form of epilepsy characterized by multiple manifestations of cognitive impairment and developmental delays1. Solving problems in EPILEPSY: 7 ≈700K prescriptions annually have seizures related to 50K Of nearly 3.4MM patients with epilepsy LGS2 30-40% Of people with LGS have dysphagia3 • Under FDA review for management of refractory patients with epilepsy on stable regimens of AEDs who experience seizure clusters. “ “≈90% of patients with refractory seizures will not interact with the historically available treatment5 ≈1.2M Epilepsy patients4 suffer from uncontrolled, refractory seizures visits caused by seizures annually6 >1M Emergency Department SYMPAZAN Oral Film (Clobazam) LIBERVANT Buccal Film (Diazepam)
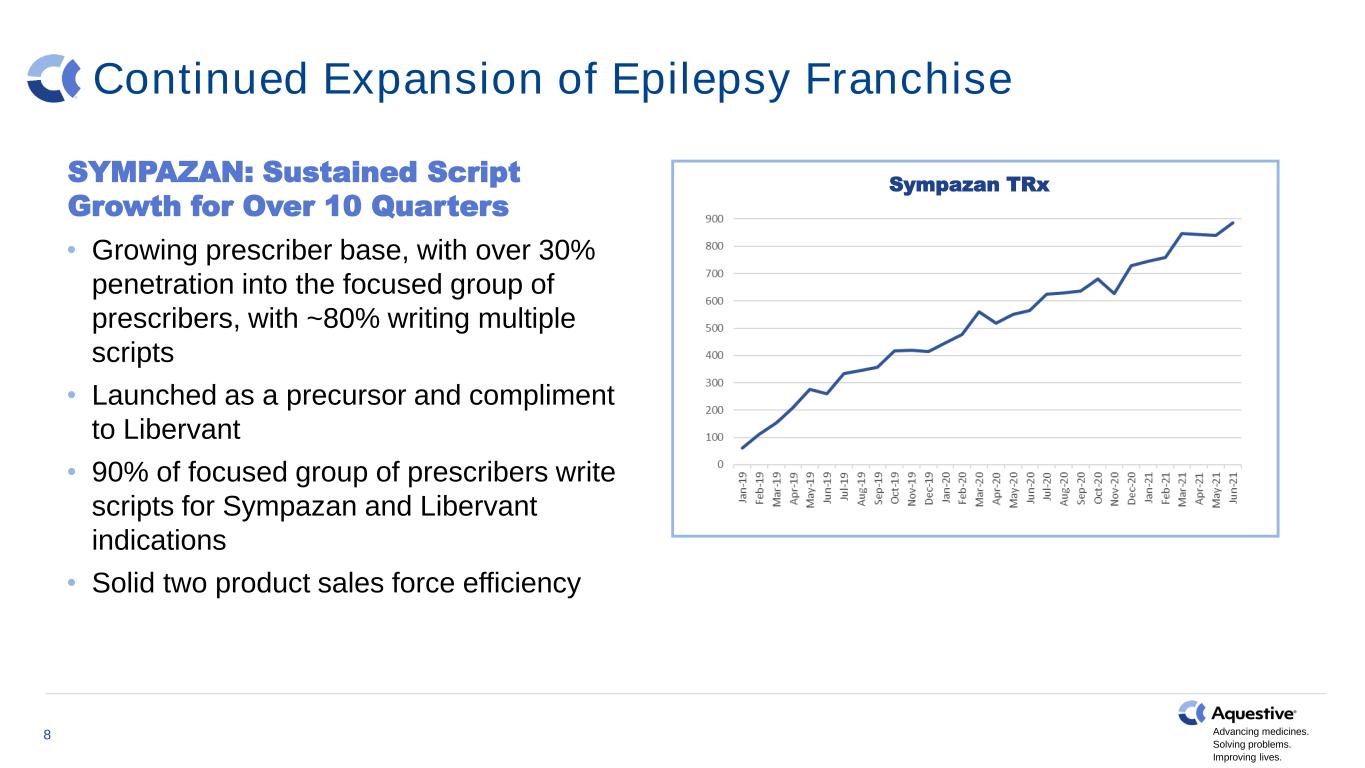
Advancing medicines. Solving problems. Improving lives. Continued Expansion of Epilepsy Franchise SYMPAZAN: Sustained Script Growth for Over 10 Quarters • Growing prescriber base, with over 30% penetration into the focused group of prescribers, with ~80% writing multiple scripts • Launched as a precursor and compliment to Libervant • 90% of focused group of prescribers write scripts for Sympazan and Libervant indications • Solid two product sales force efficiency Sympazan TRx 8

Advancing medicines. Solving problems. Improving lives. LIBERVANT: NDA Resubmission Original NDA Submission • November 2019 CRL Received • September 2020 NDA Resubmission • June 23, 2021 Expected LAUNCH • Early 2022 9 PDUFA Date • December 23, 2021

Advancing medicines. Solving problems. Improving lives. Solving Problems in ANAPHYLAXIS • Anaphylaxis is an unpredictable, severe systemic allergic reaction that is rapid in onset and potentially fatal7 • At-risk patients should always have immediate access to 2 doses of epinephrine8 • Delayed administration of epinephrine tied to increased fatalities9 • Aquestive is developing oral sublingual film formulations of epinephrine for treatment of allergic reactions (type 1), including anaphylaxis 10 ≈$1.5B market with ~3M total prescriptions13 Hospital Admissions by 500-700% in last 10-15 years11 Increased Approximately 186 225 deaths per year12 to 5% The lifetime prevalence of anaphylaxis in the U.S. could be as high as As a treating physician and researcher in anaphylaxis, I believe the film dosing could represent a significant advance in treatment for patients at risk for anaphylaxis. –David M. Fleischer, MD, FAAAAI Professor of Pediatrics Section Head, Allergy and Immunology Children’s Hospital Colorado University of Colorado Denver School of Medicine “ “ 10

Advancing medicines. Solving problems. Improving lives. Key Pipeline Milestones 2021 2022 1H 2H 1H 11 AQST-108-SF (Epinephrine) Libervant™ Oral Film (Diazepam) PDUFA Date 12/23/21 NDA resubmission 6/23/21 Continued market formation and penetration AQST-109-SF (Epinephrine) Sympazan® Oral Film (Clobazam) Initiate third PK study (1Q21) and R&D Day (March ‘21) Launch and Commercialization Initiate IND process (1H22) Pilot and pivotal PK trials (2022) Phase 1 PK study top line data and pre-IND meeting (2H21)

Advancing medicines. Solving problems. Improving lives. Financial Summary 12 Capital Adequacy • Cash on hand ($34.2 million at 6/30/21) • Results from business performance, ATM activity, and expense management provide 12 months or more of capital with additional options and supports possible launch of Libervant and pipeline activities • Debt reduced to $51.5 million; potential additional capital, at Aquestive’s option, of $10 million after Libervant FDA approval and $20 million after Libervant U.S. market access • Available shelf registration Full Year 2021 Guidance (as of August 3, 2021) • Total revenues of approximately $46 million to $48 million • Non-GAAP adjusted gross margins of approximately 70% to 75% on total revenues • Non-GAAP adjusted EBITDA loss of approximately $39 million to $42 million

Advancing medicines. Solving problems. Improving lives. Our Focus in 2021 Continue to expand in our epilepsy franchise • Focus on the FDA approval and launch of LIBERVANT • Generate continued growth in SYMPAZAN prescriptions Advance our novel epinephrine delivery platform • Complete and readout on clinical studies • Request pre-IND meeting with FDA to establish path forward • Identify additional product opportunities Continue to strengthen the capital position of the Company • Continued strong business performance to generate cash • Access available funds on potential Libervant approval and U.S. market access • Appropriate use of ATM facility • Utilize shelf registration under favorable conditions 13

Advancing medicines. Solving problems. Improving lives. 14 References CORPORATE INFORMATION , PHARMFILM® TECHNOLOGY, SYMPAZAN®, LIBERVANT™ AND EPINEPHRINE DATA 1. van Rijckevorsel. Treatment of Lennox-Gastaut syndrome: overview and recent findings Neuropsychiatr Dis Treat. 2008;4(6):1001-1019 2. Kwan and Brodie. Early Identification of Refractory Epilepsy N Engl J Med. 2000;342(5):314-319 3. Ogawa K, Kanemoto K, Ishii Y, Koyama M, Sirasaka Y, Kawasaki J, Yamasaki S. Long-term follow-up study of Lennox–Gastaut syndrome in patients with severe motor and intellectual disabilities: with special reference to the problem of dysphagia. Seizure. 2001; 10:197-202 4. Laxer KD, Trinka E, Hirsch LJ, et al. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014;37:59-70. 5. Triangle Insights Group. Synthesis of Epilepsy (ARS) Primary Research. 2017. Internal Aquestive report:unpublished. 6. Pallin DJ, Goldstein JN, Moussally JS, Pelletier AJ, Green AR, Camargo CA Jr. Seizure visits in US emergency departments: epidemiology and potential disparities in care. Int J Emerg Med. 2008;1(2):97-105. 7. Simons F.E., Clark S., Camargo C.A. Jr: Anaphylaxis in the community: learning from the survivors. J Allergy Clin Immunol 2009, 124 (2): 301–306 8. Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 Suppl):S1- S58. 9. Song TT, Lieberman P. Epinephrine in anaphylaxis: doubt no more. Current opinion in allergy and clinical immunology. 2015;15(4):323-8. 10. Shaker et al. (2020). Anaphylaxis—a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy & Clin Immunology, Vol 143, Num 4 11. Yu, J., Lin,R. The Epidemiology of Anaphylaxis. Clin Rev Allergy Immunol. 2018 Jun;54(3):366-374.doi: 10.1007/s12016-015-8503-x. 12. Borish, L., Danoff, T., Ma, L. VOLUME 133, ISSUE 2, SUPPLEMENT . doi.org/10.1016/j.jaci.2013.12.834 13. Symphony Health 2020 data on file.

Advancing medicines. Solving problems. Improving lives. Thank You
